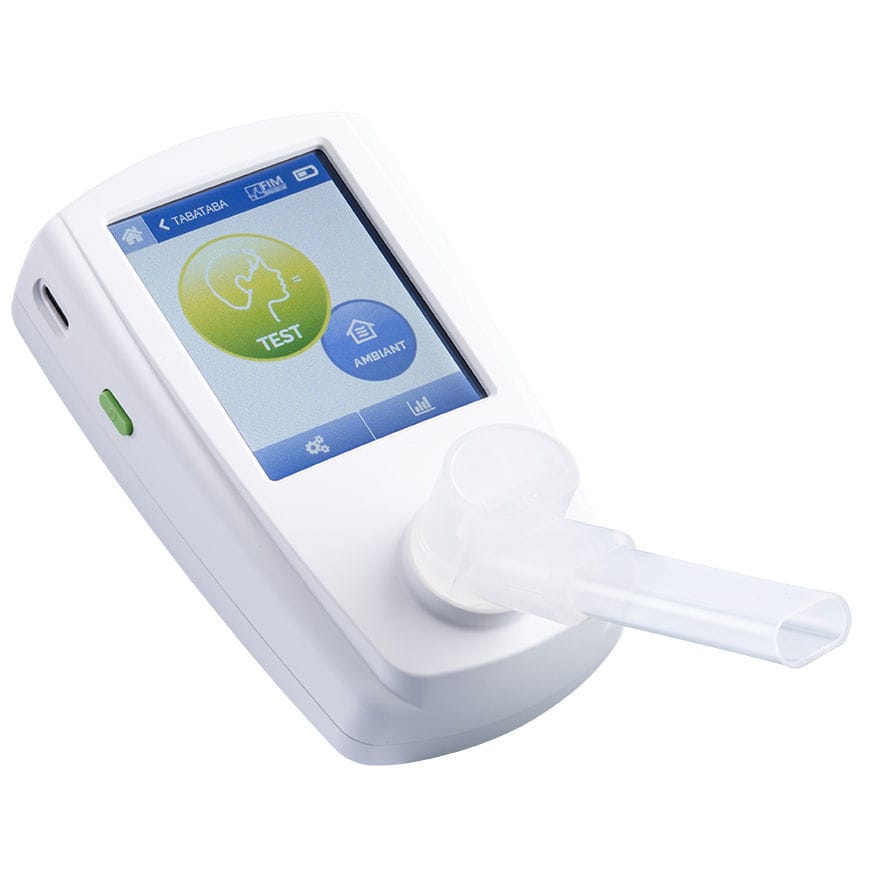
Your ally for support and monitoring of smoking cessation.
What makes the Tabataba® V3 unique
Modern, simple interface, easy to learn
Raises awareness for active and passive smoking cessation.
Calculates foetal carbon monoxide intoxication level.
Free web-based application
Export and analysis of results (CSV, PDF)
SMOKING KILLS, RAISING AWARENESS AND ACCOMPANYING YOUR PATIENTS CAN SAVE LIVES!
Always have your CO Tester to hand!
others strenghts
Mesures réalisées en quelques secondes
Accès aux informations produits et patients
Appareil entièrement paramétrable.
Export des résultats au format CSV
Suivi des résultats
Aide à l’interprétation (rapport patient PDF)
Date des dernières calibrations
Mode calibration disponible
Consommables à usage unique de faible encombrement
Features
CO measurement range: 0 to 500 ppm
Measurement tolerance: +/- 3 ppm
Rates displayed : ppm, %HBCO et %HBCO fœtal
Lithium battery: 2200 mAh / 3.7 V
Battery capacity: up to 150 measurements – 40 patients – 4 hours in ambient air
Sensor service life: 5 years
Manufacturer warranty: 2 years
CE marking (0459), medical class Im, complies with regulation 2017/745
This device is intended for healthcare professionals
Medical screening device for :
Occupational Health doctors, nurses, associations (cancer leagues etc.), maternity wards, midwives, school health, health examination centres, lung specialists, allergists, oncologists, general practitioners, pharmacies, insurance companies and any other profession involved in prevention.
 USDEnglish
USDEnglish TRYTurkish
TRYTurkish







