A breath of fresh air, the Spirolyser® Q13® is unique
Single-use Fleisch type sensor, high technology.
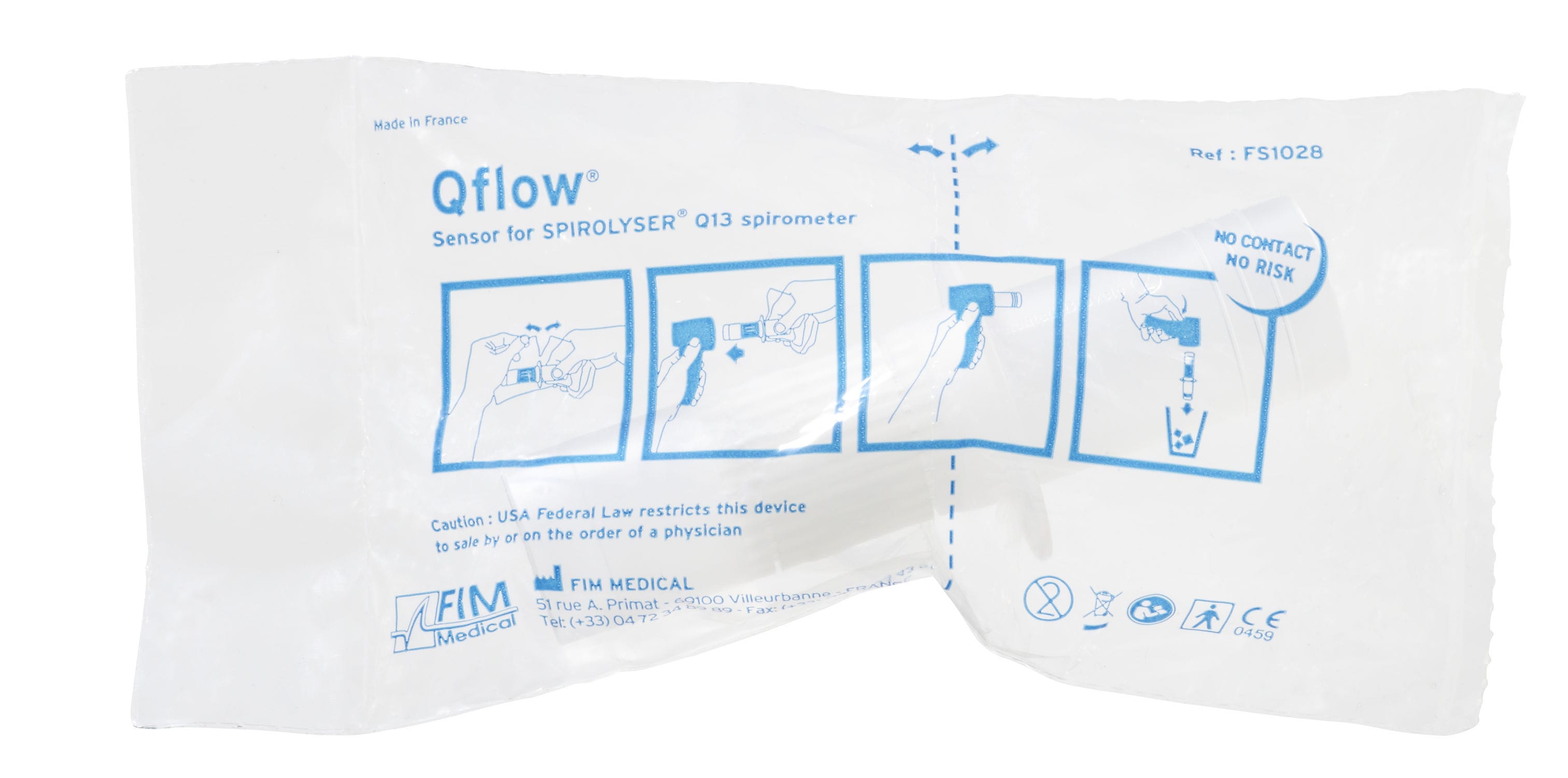
Concept No Contact No Risk : No disinfection, no calibration, no cross-contamination.
Weather station integrated in the spirometer indicating temperature, pressure and humidity.
Multitude of predicted values possible.
Concept “NO CONTACT NO RISK”.
No disinfection, no calibration, no cross-contamination.
Slow Vital Capacity (VC)
Forced Vital Capacity (FVC) / Post-medication
Maximal Voluntary Ventilation (MVV)
Others strengths
Plug & Play, quick and easy to use
Qflow®: Fleisch type pneumotachograph
Concept « No Contact, No Risk »
Integrated weather station
Tests: Slow Vital Capacity, Forced Vital Capacity, Post-medication, Maximal Voluntary Ventilation (MVV)
Predicted Values: Dejsomritrutai 2000, Eccs/Polgar, Eccs/Zapletal, GLI, ITS (Crapo), Knudson, Nhanes III…
Help in interpreting results: Occupational Health, Professor Perdrix, GOLD…
Measurement accuracy: VC, FVC, VEMS : <± 3% ; PEF: <± 10% ; FEF25-75 : <± 5% ; MVV : <± 10%
Amusing incentive videos, demo videos
Secure database
Comparison of tests and export and available
Compatible with a lot of professional software
Lead length: 3 metres
Installation and user training videos available on request
Manufacturer warranty : 2 years
CE (0459) Marking, Medical Class IIa
This device is intended for healthcare professionals

 USDEnglish
USDEnglish TRYTurkish
TRYTurkish